Recently, the joint teams of Peking University and Suzhou University have made major breakthroughs in the preparation and performance research of sub-nanometer metal materials. They synthesized Pt and Pt-based alloy nanowires with diameters of only a few atomic layers through high-temperature oil phase and applied them as oxygen reduction reaction (ORR) electrocatalysts that limit the commercialization of fuel cells, exhibiting ultra-high electrocatalysis. Active and excellent stability. In addition, the conclusion of this work has broken through the traditional understanding that the small-size Pt-based nanomaterials are too insensitive to the reaction of ORR intermediates, and have pioneered the application of subnanometer one-dimensional materials for electrocatalytic reactions. Guo Shaojun, a professor at Peking University’s College of Engineering, is the author of the newsletter. The results of this collaboration were published in the latest issue of the internationally recognized academic journal Science Advances (link: http://advances.sciencemag.org/content/3/2/e1601705). Fuel cells, especially Proton exchange membrane fuel cells (PEMFCs), are considered to be the best productivity devices in the future due to their high efficiency, environmental friendliness, and high degree of modularity, and have also been evaluated by many science and technology magazines. The top ten hi-tech in the eleventh century. As early as 2014, the Japanese car giant Toyota has developed a small car called Mirai, which uses PEMFCs as its main power source. Although PEMFCs have achieved unprecedented development over the past few decades, their further commercial application is still limited by the high Pt loading used to catalyze their cathode ORR reactions. Therefore, there is an urgent need to develop low cost and high performance. ORR electrocatalytic material. In the early solutions, the researchers were caught in a dilemma: in order to increase the utilization of Pt in precious metals, the size of Pt catalysts needs to be continuously reduced; and small-size Pt adsorbs too strongly on ORR reaction intermediates, which is not conducive to specific activity. The improvement. Based on the above two factors, it was found that the mass specific activity of Pt particles with a size of about 3 nm is the highest (that is, the activity per unit mass of Pt), which obviously cannot meet the requirements for PEMFCs to go to market. How to further increase the mass-specific activity of Pt has become a core issue in this field. The research team found that previous studies were mostly confined to zero-dimensional Pt polyhedrons, and the dilemma mentioned above applies to one-dimensional nanowire structures as well. In order to solve this scientific problem, the research team reported a universal method for preparing sub-nanowires based on high-temperature oil phase synthesis. This method can not only obtain nanowires with a diameter of only 4 to 5 atomic layers, but also extend to binary or even ternary alloys. Unlike the zero-dimensional polyhedron structure, the sub-nanowire structure not only greatly improves the atomic utilization of Pt, but also improves the area specific activity. At 0.9 V, the mass and area specific activity of the PtCoNi ternary alloy nanowires were as high as 4.2 A/mg and 5.1 mA/cm2, respectively, which were 32 and 27 times higher than those of commercial platinum catalysts, respectively. Density functional calculation simulation results indicate that the high-density pure Pt(111) crystal plane on the sub-nanometer line is the main active site of ORR, and the compressive stress from the inner platinum-based alloy core optimizes the adsorption of ORR intermediates by the outer Pt shell. Bond energy, which facilitates the reaction. In addition, this method is also suitable for the mass production of high-performance nanocatalytic materials. The work was completed in close cooperation with the three units of Peking University, Suzhou University and California State University. Guo Shaojun and Huang Xiaoqing were the authors of the papers. The project was supported by Peking University’s Center for Engineering Science and Technology Innovation Center, the Ministry of Science’s key research and development program, and the National Natural Science Foundation. Free Running Wire Thread Insert Free running wire thread inserts can be used to strengthen threads, giving applications a longer life. Each insert sharing the load over the entire bolt and hole, improving holding or pull out resistance. With a Wire Thread Insert installed, a more even distribution of load and stress can be achieved. Free running wire thread inserts are generally made of type 304(18-8) stainless steel wire rolled into a diamond shape cross-section. Free Running Wire Thread Insert,Hardware Fastener Wire Thread Insert,Stainless Steel Wire Thread Repair Insert,Stainless Steel Wire Thread Inserts Shenyang Helisert Technology Co., Ltd , https://www.helisert.com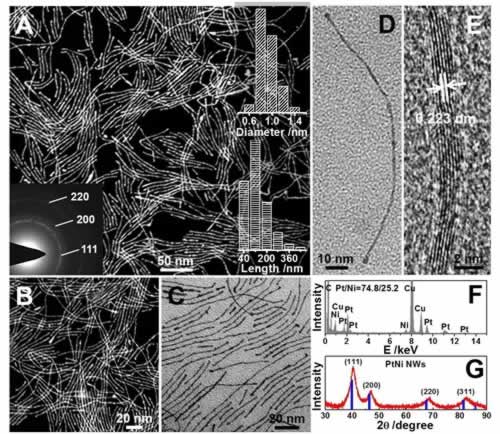
Fine Structure Characterization of Pt-based Nanowires 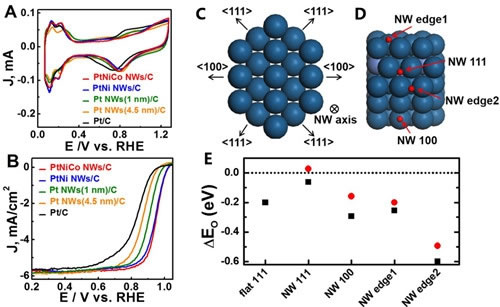
Electrochemical Characterization and DFT Simulation of Pt-based Nanowire Catalysts